Secure a Drug
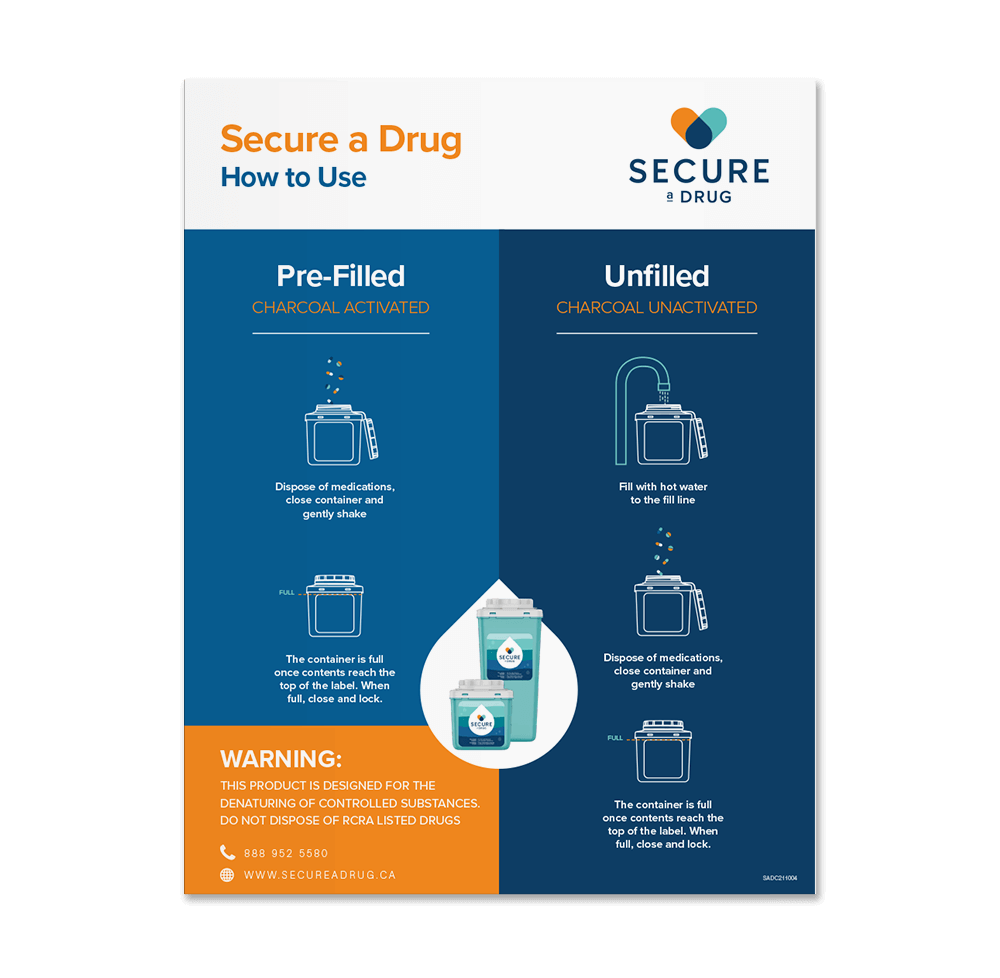
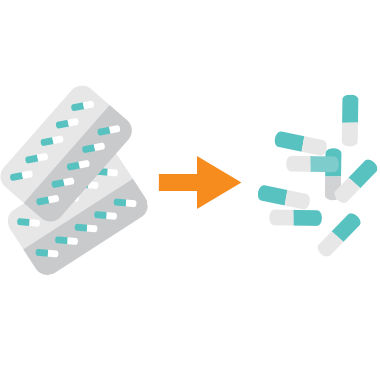
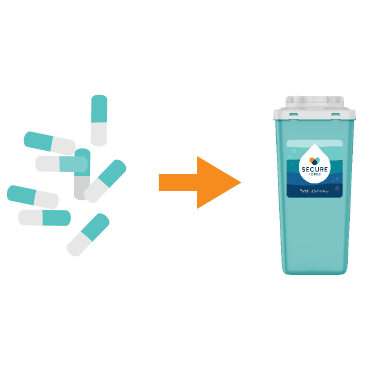
The leading hospital and pharmacy solution for the safe disposal and deactivation of medications and controlled substances. Secure a Drug neutralises pharmaceuticals in just three steps, allowing for the permanent diversion of left over medications
Drug Disposal designed for Healthcare Professionals
Keeping healthcare professionals at the forefront of our focus, we set out to design a product that was both simple and effective for medication neutralization and disposal. Our goal was to develop a solution for drug diversion prevention that would be easy for health professionals to integrate into their already busy schedules, with the level of prioritisation that is critical in eliminating accessibility to unused medications and preventing diversion.
CARE FOR THE CARERS
One in Ten healthcare workers experience some type of substance use disorder. Protect your fellow healthcare workers from unsafe drug diversion. Keeping our clinicians protected.
ELIMINATE WATERWAY POLLUTION
Fish and pharmaceuticals really don’t vibe! Get compliant with the EPA’s sewer-ban of hazardous pharmaceuticals, protecting our waterways and safe drinking water with safe drain-free disposal of pharmaceuticals.
ELIMINATE GROUNDWATER LEACHING
Eliminate incorrect treatment and groundwater leaching. Safely dispose of DEA regulated pharmaceuticals, Fentanyl, Hydrocodone, Oxycodone and controlled substances per schedules I-V

Let’s Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
 Daniels
Daniels  HOW IT WORKS
HOW IT WORKS