Biomedical Waste Bin Cleanliness in a Pharmacy Clean Room

In a CDC post on Healthcare-Associated Infections (HAIs) in the United States, it was noted that a failure in a pharmacy clean room resulted in 753 patients being infected with meningitis, causing 64 deaths in 20 states. This is one area of a hospital where contamination risk should not be taken lightly. (source)
So when a Pharmacy Clean Room fails to meet the acceptable levels of microbes allowed – how is that problem solved?
In this blog:
- The Challenge: Failed Clean Room Test
- Our Approach: Remove Outdated Waste Containment Systems
- The Solution: Implement a Smarter Waste Container
- The Results: No Further Clean Room Test Failures
“It is essential that perioperative nurses, environmental services personnel, anesthesia technicians, and anesthesia professionals properly disinfect environmental surfaces to prevent HAIs.”
After two routine tests, a Pharmacy Clean Room failed to meet the acceptable levels of microbes allowed.
Prior to the first test, the surfaces and ducts of the Pharmacy Clean Room were thoroughly cleaned; however, the test was still not successful.
The second assessment round prompted the testing of the reusable biomedical waste containers in the room. The results revealed extremely high microbial counts on the surface of the containers. The microbial count was determined through an ATP test, the process of rapidly measuring actively growing microorganisms through detection of adenosine triphosphate (ATP). The goal of an ATP test in this scenario was to reduce the ATP count to a target-level of <250 relative light units; the test failed with the average of the highest count being 14,844 – exceeding safe levels.

Our solution to improving the cliinical hygiene of the Pharmacy Clean Room was our bagless, hands-free biomedical waste container: The Medismart.
Where other biomedical waste containers are supplied with multiple units “nested” within each other which entraps moisture in the bottom of the bin and enables microbial growth – Daniels’ Medismart container is supplied as a single sealed unit eliminating that risk.
All Daniels’ containers undergo a thorough washing and decontamination process after each use. This patented robotic system is fully automated to decant, wash, sanitise and dry the collectors before redeployment; Daniels’ Washsmart system has been extensively researched, tested and independently verified as achieving a level of sterilisation 4x higher than required by the CDC in the United States.
Infection prevention must be a priority in healthcare waste management strategy – and that includes reducing overall touches during the dispsoal process. Daniels’ Medismart enables hands-free disposal with foot-pedal activation, eliminating surface touches.
An ideal solution for Pharmacy Clean Rooms which require scrupulous decontamination to an ISO standard, Medismart stands alone in its benchmark of microbiological efficacy. Enquire about the Medismart

Microbial count is determined through an ATP test, the process of rapidly measuring actively growing microorganisms through detection of adenosine triphosphate (ATP). The goal of an ATP test in this scenario was to reduce the ATP count to a target-level of <250 relative light units.
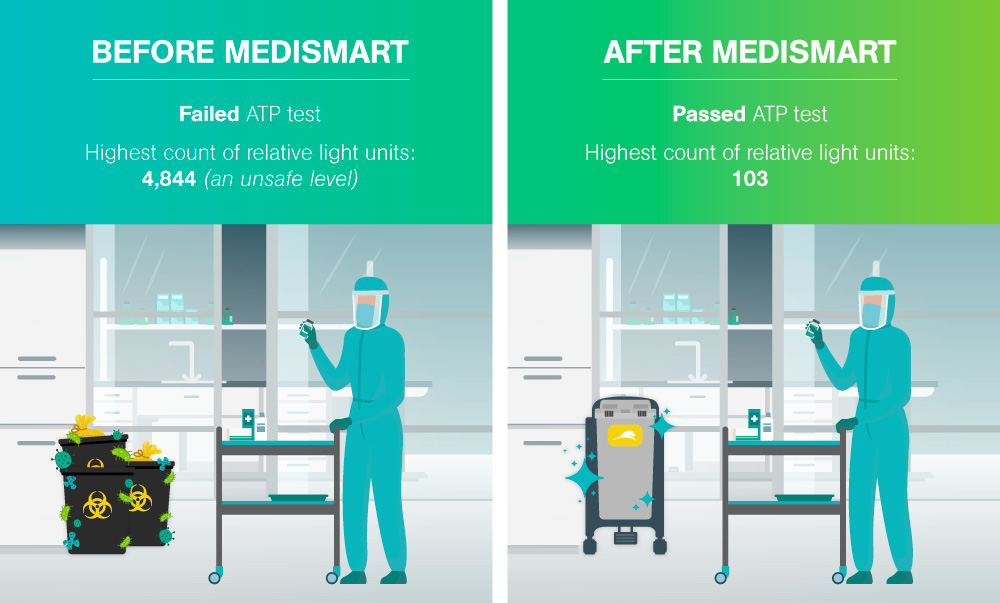
No further work-surface tests failed and since the Medismart was implmented in August 2015, no failures in the Pharmacy Clean Room microbe tests have occurred.
Learn more about the Medismart
Authors Tyler Weaver, Josh Guinter (Childrens hospital of Philadelphia), and microbiologist Terry Grimmond presented their case study “Resolving microbial contamination of reusable waste bins in a pharmacy clean-room” at the Association for the Healthcare Environment (AHE) conference in Pittsburg PA. A recap published by one of the authors can be found here.
Let's Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.